Acyclovir Suspension – Oral Dispenser
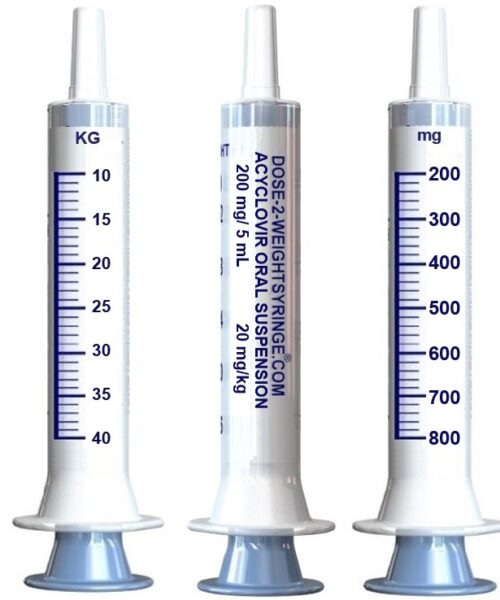
Pediatric Dosing Information
The current Prescribing Information (PI), for the administration of acyclovir oral suspension (200mg/5mL) for pediatrics recites; for children 2 years or older and less than 40 kg for the treatment of Chickenpox, Herpes Simplex Labialis, Zoster and Varicella – 20 mg/kg per dose orally 4 times daily (80 mg/kg/day) for 5 days.1 The PI and product label further describes the dosage of the acyclovir oral suspension as 200 mg of acyclovir in each teaspoonful (5 mL).
Conversely the PI does not describe the extrapolation of the 20 mg from the 5 mL suspension nor the procedures for apportioning of 10% of the teaspoon (20mg) for the subsequent conversion to an individual kilogram patient dose as prescribed.
This dosing regimen provides an elevated risk of error and inaccurate dosing, due to calculations and measurements procedures required to perform by health care providers.
Moreover, dozens of studies conclude that a household teaspoon is not an adequate measuring device.2 According to the American Academy of Pediatrics (AAP) and the US Food and Drug Administration (FDA), among other health agencies, non-standard kitchen spoons are not recommended as a dosing apparatus for medicinal solutions. 2,3,4,5

