The patented Safe-2-Dose™ Integrated Dosing Container is the next generation Infants elixir dispenser providing enhanced medication dosing accuracy and user convenience. The caregiver need only disabl

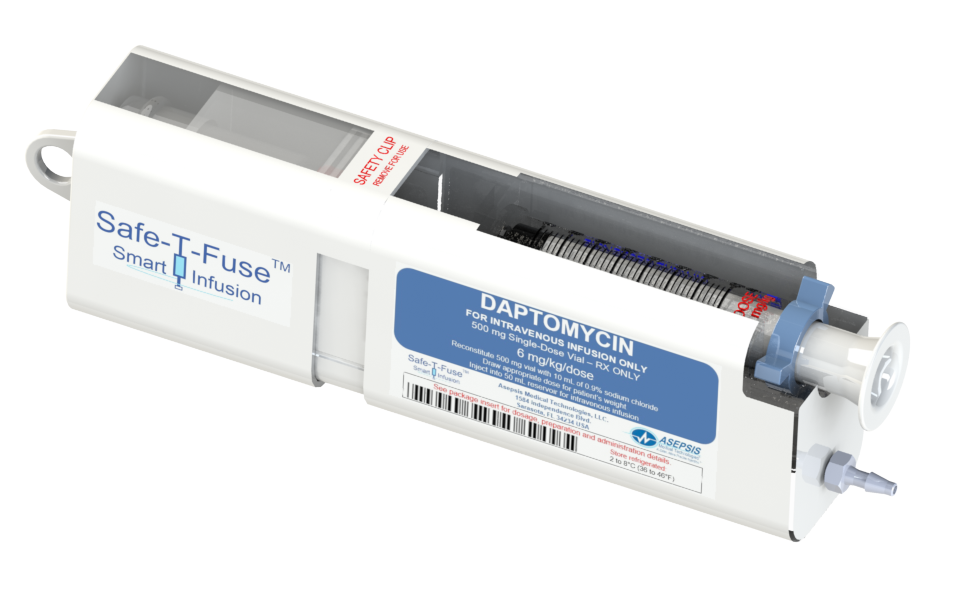
The Safe-T-Fuse™ home infusion system is a calibrated, tamper proof system that simplifies the preparation and administration of home medication infusion. The infusion canister is designed to accept industry standard vials that are inserted within a holding mechanism within the devices’ sleeve cap. Once the sleeve cap is engaged to the body of the Safe-T-Fuse™ the sleeve cap and vial cannot be removed or accessed in anyway.
A tamper resistant clip prevents the sleeve cap containing the vial from being further depressed and engaged. Once removed the sleeve cap may be fully depressed allowing for the vial to be pierced and the subsequent bi-directional flow between the solutions contained within the reservoir of the Safe-T-Fuse™ and the medication within the vial.
The established bi-directional flow allows for a pre-determined amount of solution to reconstitute the vial of medication, or allows for a vial containing a medicated solution to be transfer directly or pre-metered dosed into the Safe-T-Fuse™ infusion reservoir.
The user’s actions are controlled by active safety features that virtually prevent the incorrect preparation procedures, thus further preventing MAE’s and ADE’s. After depressing and engaging the sleeve cap the user simply would have to pull back and forth the plunger to a pre-set stop and turn a dial.
Not available in all territories.
The Safe-T-Fuse™ is a trademark of Asepsis Medical Technologies, LLC., and covered by two or more patents, including; U.S. Patent 9,433,726 and U.S Patent 11,058,606 as well as Patents Pending.
Video
Technical Video
Latest News
Asepsis Announces The Grant Of Mexico Patent No. 384,129
Asepsis Medical Technologies® is proud to introduce the latest addition to the Calibrated Drug Delivery™ (CCD) platform, the Multi-Dose Safe-2-Dose® syringe. The Multi-Dose Safe-2-
Asepsis Announces the Grant of Canadian Patent No. 3,006,218
Asepsis Medical Technologies® is proud to announce their latest Canadian Patent Grant 3,006,218 of August 24, 2021 – for the multi-dose oral Dose-2-Weight Syringe®. The Dose-2-Weight Sy
Asepsis announces the US Patent Grant 11,058,606
Asepsis Medical Technologies announces the USPTO issuance of Patent Application Number 11,058,606 for its Safe-T-Fuse™ drug infusion system. The Safe-T-Fuse™ is an industry unique infusion contai
Asepsis Announces the Grant of Mexico Patent MX/a/2017/012034
The Safe-T-Syringe® is designed to deliver the highest standard of patient care via syringe. Its medication calibrated indicia provide enhanced accuracy, convenience and safety through-out preparation
Asepsis Announces the Grant of U.S. Patent 10,857,299
Asepsis Medical Technologies® is proud to introduce the latest addition to the Calibrated Drug Delivery™ (CCD) platform, the Multi-Dose Safe-2-Dose® syringe. The Multi-Dose Safe-2-Dose® syringe provid
Asepsis Announces the U.S. Patent Grant No. 10,625,026
Asepsis Medical Technologies announces the U.S. Patent Grant for the Momenta-Dose™ forcé actuated injection device. The Momenta Dose™ is designed to administer rapid small volume subcutaneous or intra
