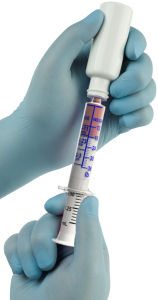
Asepsis Medical Technologies announces the issue of United States Patent 9750883 for its Oral-Dose-2-Weight Syringe® and Parenteral-Dose-2-Weight Syringe®. The Asepsis’ Calibrated Drug Delivery™ platform is designed to enhance dosing accuracy and eliminate crucial procedural tasks that are the leading cause of error during the preparation and administration of oral and injectable medications via syringe.