The patented Safe-2-Dose™ Integrated Dosing Container is the next generation Infants elixir dispenser providing enhanced medication dosing accuracy and user convenience. The caregiver need only disabl
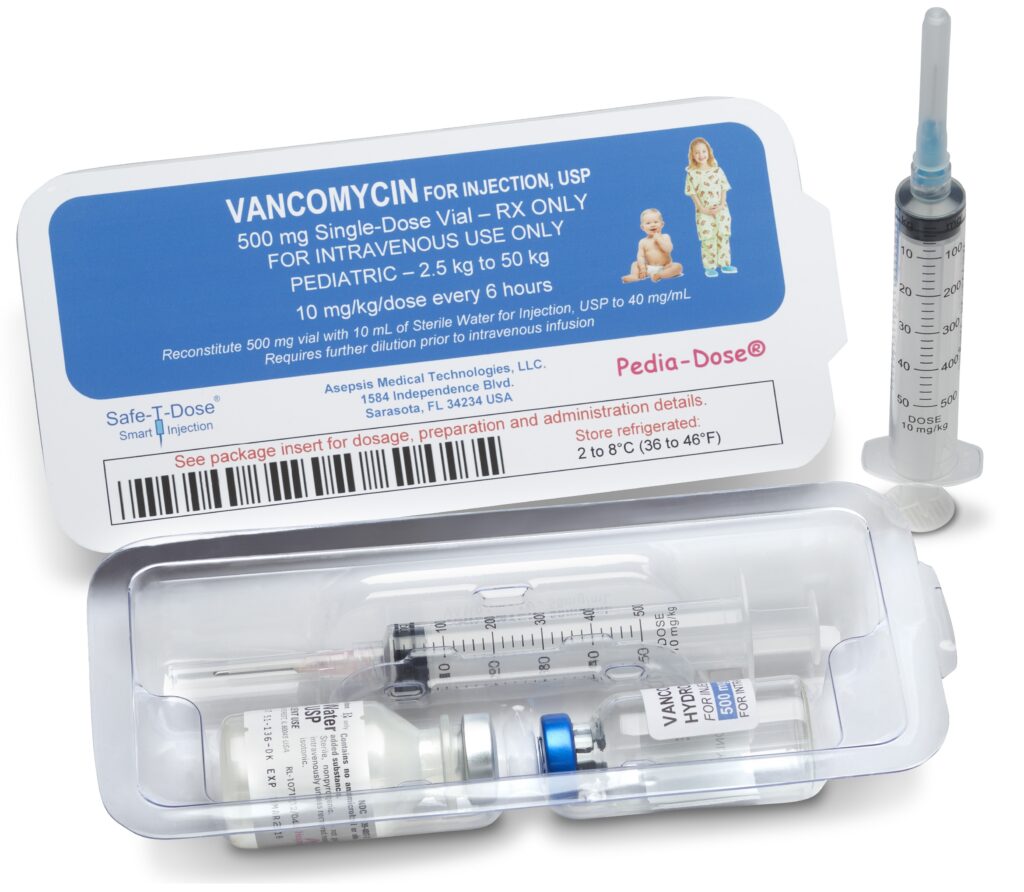
Pedia-Dose® is specifically designed to offer reduced dosage and volume medications for neonates and pediatric patients. The design of Pedia-Dose® specifically for pediatrics is extremely valuable as it contains all components necessary to provide a precise and accurate injection while significantly mitigating the possibility of a medical administered error (MAE).
There have been multiple published studies regarding the difficulties and errors with administering small doses of medication, primarily for infants and small children. Exacerbating the issue is the fact that small doses for young patients are often prepared from stock of less than 0.1mL in size, yet the equipment does not permit the accurate measurements of volumes that small.
A study from the Canadian Medical Association Journal on this subject found 17.5% of children and babies need preparations of less than 0.2 mL and concluded a substantial source of dosing error involved more than 25% of the children studied. Adverse drug event (ADE) rates are generally the highest among children up to age 5.
Pedia-Dose® utilizes a uniquely designed Safe-T-Syringe® that is comprised of a calibrated reduced volume barrel with multiple correlating indicia. This design provides an enhanced lower incremental dosing, while in parallel extending the indicia display of the Safe-T-Syringe® for precise accuracy in dosing of the medication. The custom indicia may also be designed to target individual patient weight class or body surface area for ultra-sensitive or potent medications, further increasing the accuracy of preparation and administration.
The unique design of the Safe-T-Syringe® indicia for the Pedia-Dose® also enables the downsizing of the medication vial to individual patient class, weight, BSA or other feature. This “designed dosage” to individual patient class creates tremendous cost savings versus the typical waste of medication from traditional dose products. In addition, the reduction in waste offers a decrease in environmental impact and disposal costs.
Not available in all territories
The Safe-T-Dose® and Pedia-Dose® are registered trademarks of Asepsis Medical Technologies, LLC., and covered by one or more patents, including; U.S. Patent 9,192,723, U.S. Patent 9,302,050, U.S Patent 9,750,883, U.S. Patent 10,857,299 and International Patents; AU2013222819, AU2016269509, AU2018232988, CA2862633, CA3006218, MX351645, MX384,129, JP2015-508321 and Patents Pending.
Latest News
Asepsis Announces The Grant Of Mexico Patent No. 384,129
Asepsis Medical Technologies® is proud to introduce the latest addition to the Calibrated Drug Delivery™ (CCD) platform, the Multi-Dose Safe-2-Dose® syringe. The Multi-Dose Safe-2-
Asepsis Announces the Grant of Canadian Patent No. 3,006,218
Asepsis Medical Technologies® is proud to announce their latest Canadian Patent Grant 3,006,218 of August 24, 2021 – for the multi-dose oral Dose-2-Weight Syringe®. The Dose-2-Weight Sy
Asepsis announces the US Patent Grant 11,058,606
Asepsis Medical Technologies announces the USPTO issuance of Patent Application Number 11,058,606 for its Safe-T-Fuse™ drug infusion system. The Safe-T-Fuse™ is an industry unique infusion contai
Asepsis Announces the Grant of Mexico Patent MX/a/2017/012034
The Safe-T-Syringe® is designed to deliver the highest standard of patient care via syringe. Its medication calibrated indicia provide enhanced accuracy, convenience and safety through-out preparation
Asepsis Announces the Grant of U.S. Patent 10,857,299
Asepsis Medical Technologies® is proud to introduce the latest addition to the Calibrated Drug Delivery™ (CCD) platform, the Multi-Dose Safe-2-Dose® syringe. The Multi-Dose Safe-2-Dose® syringe provid
Asepsis Announces the U.S. Patent Grant No. 10,625,026
Asepsis Medical Technologies announces the U.S. Patent Grant for the Momenta-Dose™ forcé actuated injection device. The Momenta Dose™ is designed to administer rapid small volume subcutaneous or intra

