The patented Safe-2-Dose™ Integrated Dosing Container is the next generation Infants elixir dispenser providing enhanced medication dosing accuracy and user convenience. The caregiver need only disabl

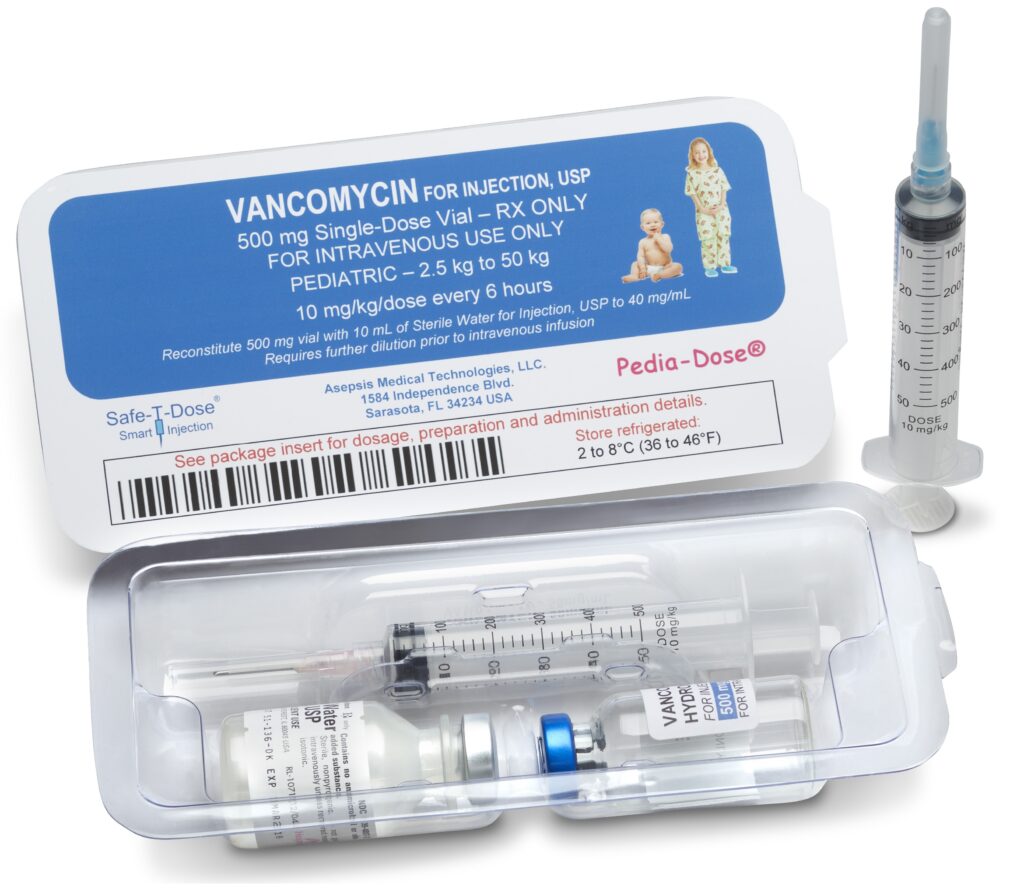
The Safe-T-Dose® is designed to offer the same combinatorial and passive safety features of the Pedia-Dose® yet contains standard non reduced dosage medication.
The Safe-T-Dose® may also be deigned to contain either a standard Safety-T-Syringe® dosage to weight indicia, or a patient specific indicia comprised of a targeted patient group, weight range, BSA or other feature.
Similar to the Pedia-Dose®, the Safe-T-Dose® eliminates critical procedural steps and methods that are the leading causes of medication errors. Both products replace these procedures with passive safety systems that complete the preparation and administration of the medication in the same fashion without any modification.
The Safe-T-Dose® would contain a particular medication and the correlating safety syringe, a Package Insert for the medication; diluent(s) with the correlating calibrated diluent syringe for the reconstitution of the medication.
The features of the Safe-T-Dose® and Pedia-Dose® completely remove multiple hands on requirements for the consolidation of the correct diluent(s) for the medication and route of administration, the calculation and correlation of the accurate amount of diluent for the medication, the correct needle for the route of administration and the calculation of the medication’s dosage to weight, BSA or other feature.
Not available in all territories
The Safe-T-Dose® and Pedia-Dose® are registered trademarks of Asepsis Medical Technologies, LLC., and covered by one or more patents, including; U.S. Patent 9,192,723, U.S. Patent 9,302,050, U.S Patent 9,750,883, U.S. Patent 10,857,299 and International Patents; AU2013222819, AU2016269509, AU2018232988, CA2862633, CA3006218, MX351645, MX384,129, JP2015-508321 and Patents Pending.
Latest News
Asepsis Announces The Grant Of Mexico Patent No. 384,129
Asepsis Medical Technologies® is proud to introduce the latest addition to the Calibrated Drug Delivery™ (CCD) platform, the Multi-Dose Safe-2-Dose® syringe. The Multi-Dose Safe-2-
Asepsis Announces the Grant of Canadian Patent No. 3,006,218
Asepsis Medical Technologies® is proud to announce their latest Canadian Patent Grant 3,006,218 of August 24, 2021 – for the multi-dose oral Dose-2-Weight Syringe®. The Dose-2-Weight Sy
Asepsis announces the US Patent Grant 11,058,606
Asepsis Medical Technologies announces the USPTO issuance of Patent Application Number 11,058,606 for its Safe-T-Fuse™ drug infusion system. The Safe-T-Fuse™ is an industry unique infusion contai
Asepsis Announces the Grant of Mexico Patent MX/a/2017/012034
The Safe-T-Syringe® is designed to deliver the highest standard of patient care via syringe. Its medication calibrated indicia provide enhanced accuracy, convenience and safety through-out preparation
Asepsis Announces the Grant of U.S. Patent 10,857,299
Asepsis Medical Technologies® is proud to introduce the latest addition to the Calibrated Drug Delivery™ (CCD) platform, the Multi-Dose Safe-2-Dose® syringe. The Multi-Dose Safe-2-Dose® syringe provid
Asepsis Announces the U.S. Patent Grant No. 10,625,026
Asepsis Medical Technologies announces the U.S. Patent Grant for the Momenta-Dose™ forcé actuated injection device. The Momenta Dose™ is designed to administer rapid small volume subcutaneous or intra

